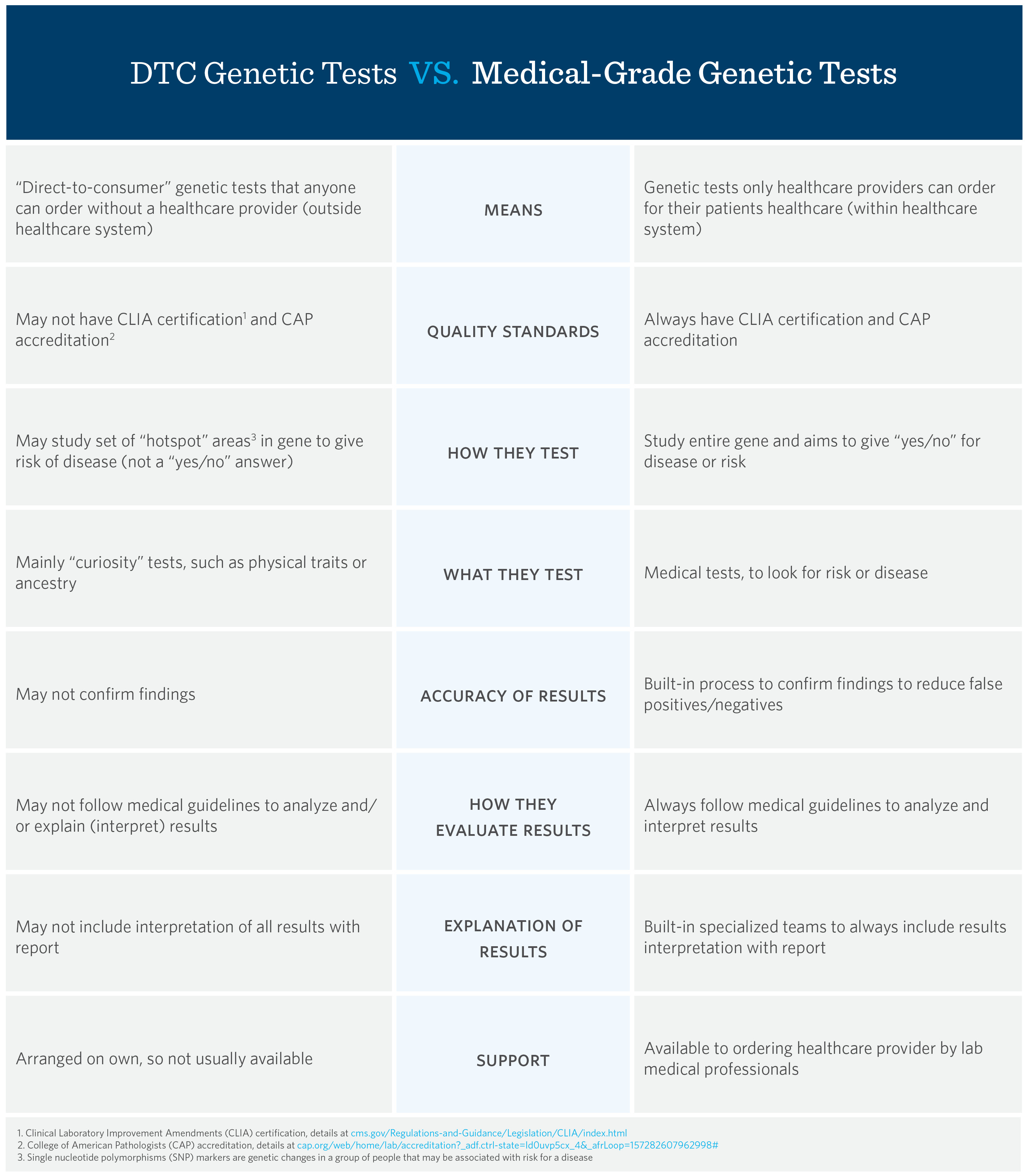
How are Direct-to-Consumer and Medical-Grade Genetic Tests Different? - View Blog Post - Ambry - Blog | Ambry Genetics

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Proficiency Testing Regulations Related to Analytes and Acceptable Performance