
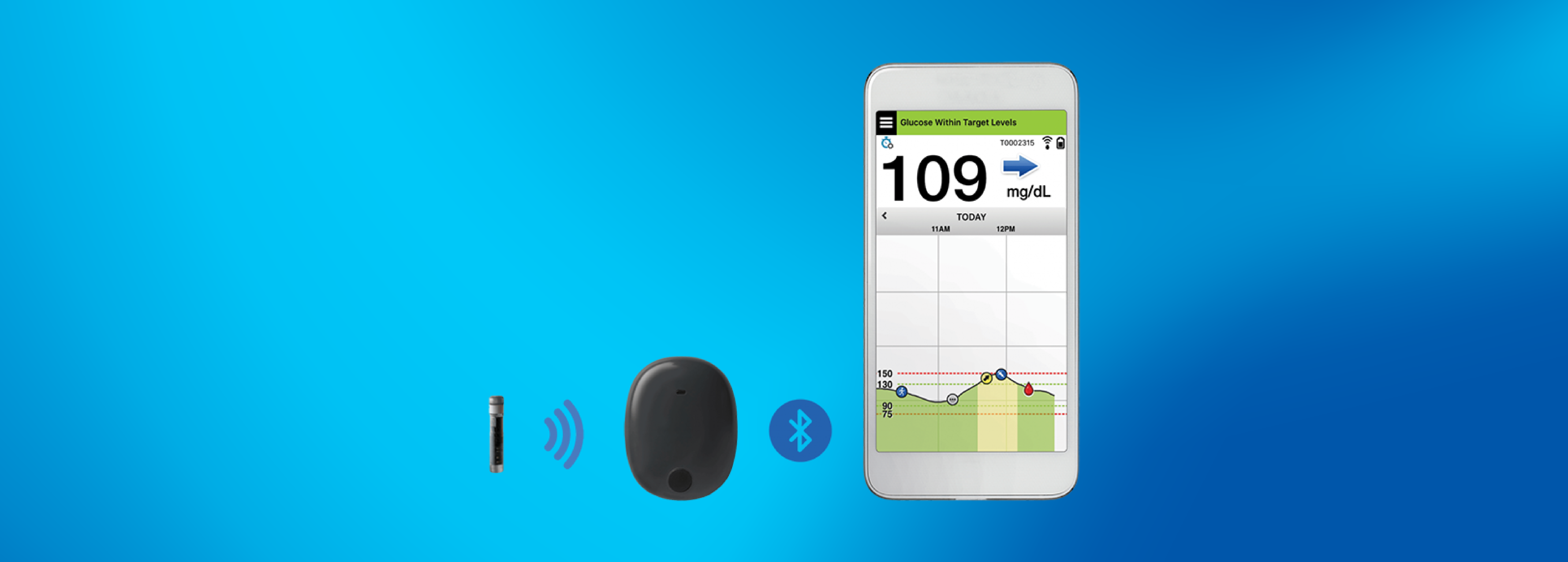
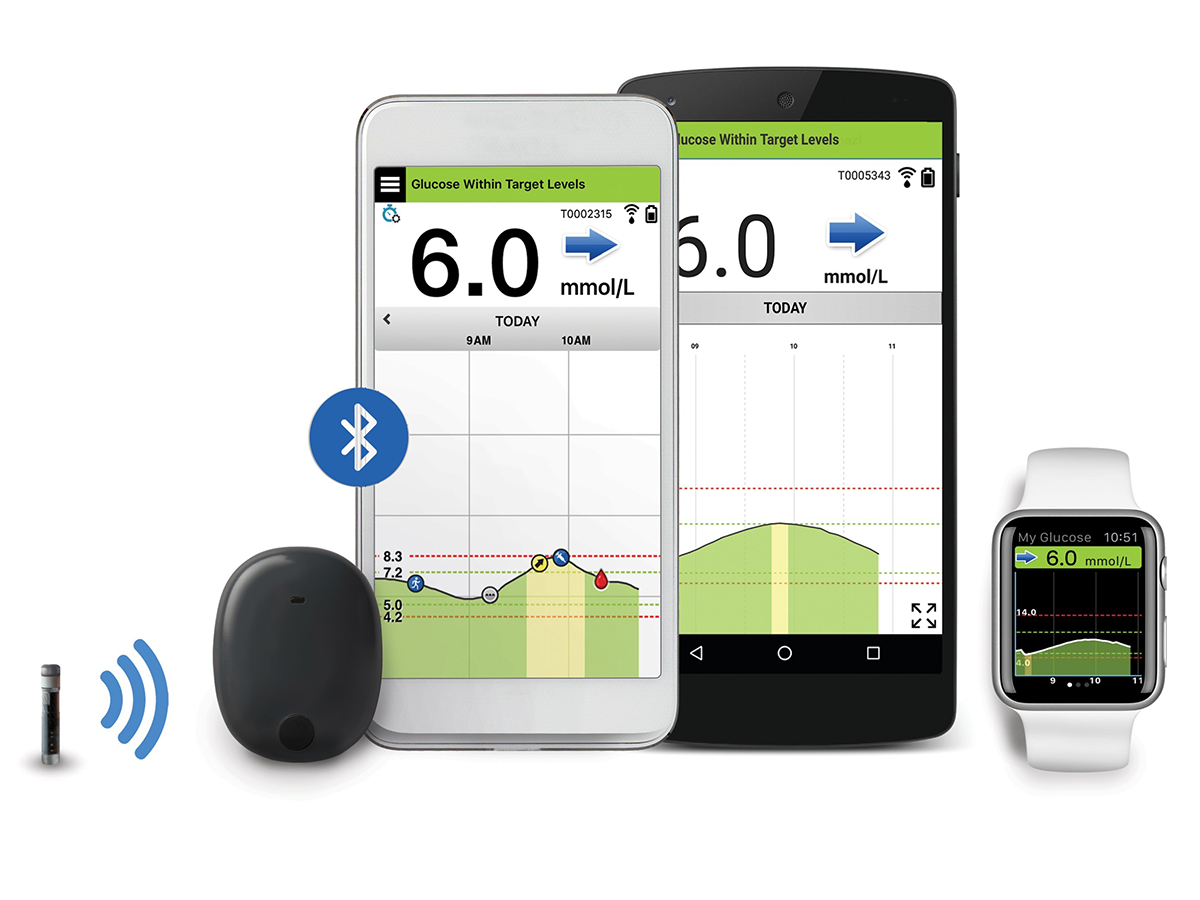
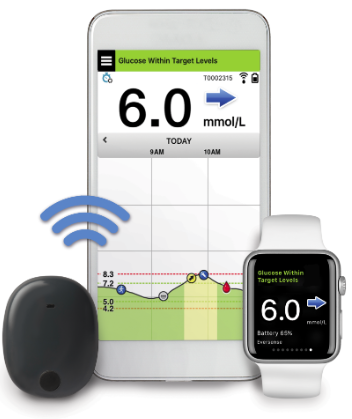
Ascensia Diabetes Care and Senseonics Announce 'The CGM for Real Life' Campaign to Raise Awareness of How Long-Term Eversense E3 Empowers People with Diabetes

Ascensia Diabetes Care and Senseonics Announce 'The CGM for Real Life' Campaign to Raise Awareness of















.jpg)


![6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider 6-Month Glucose Implant Sensor Receives CE Mark Approval [video]|Health Tech Insider](https://i0.wp.com/healthtechinsider.com/wp-content/uploads/Eversense_CGM_with_video_600x275.jpg?fit=600%2C276&ssl=1)