Senseonics Receives FDA Approval To Expand Eversense CGM Certification To Nurse Practitioners, PAs | Medical Product Outsourcing

Eversense Implantable Glucometer Gets FDA Approval as Alternative to Finger Tests - 20451 Seneca Meadows Pkwy, Germantown, MD 20876, USA

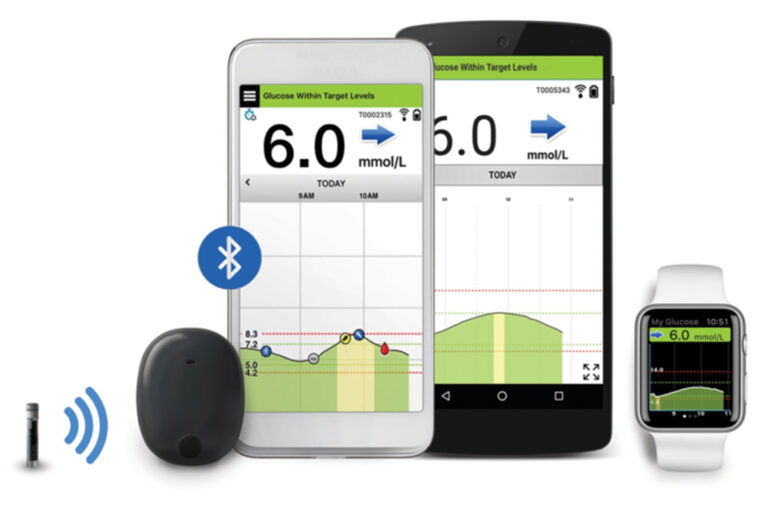
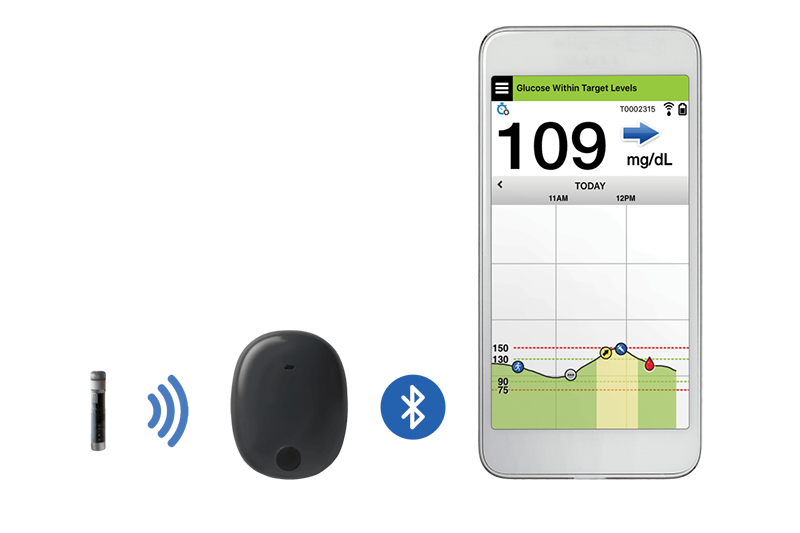
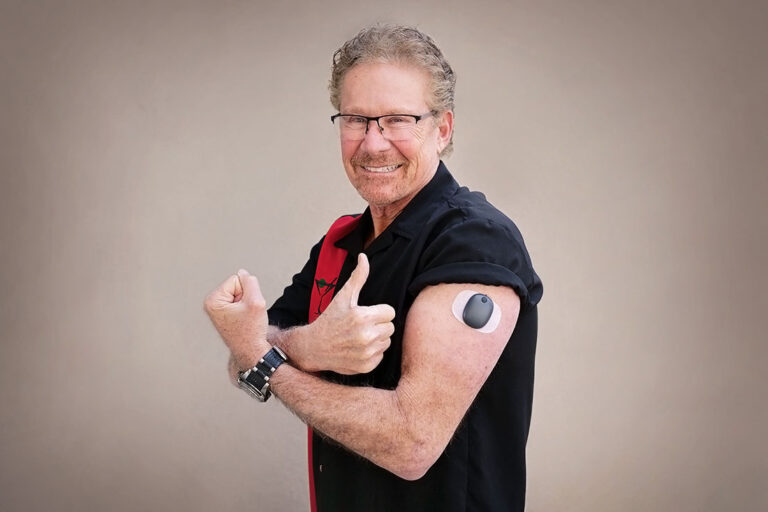
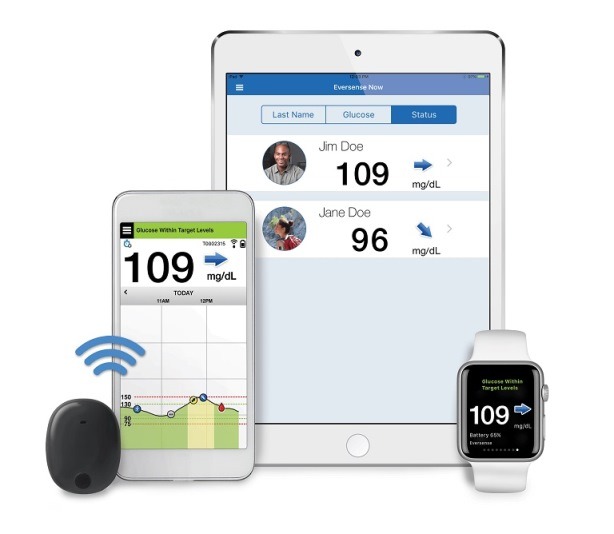
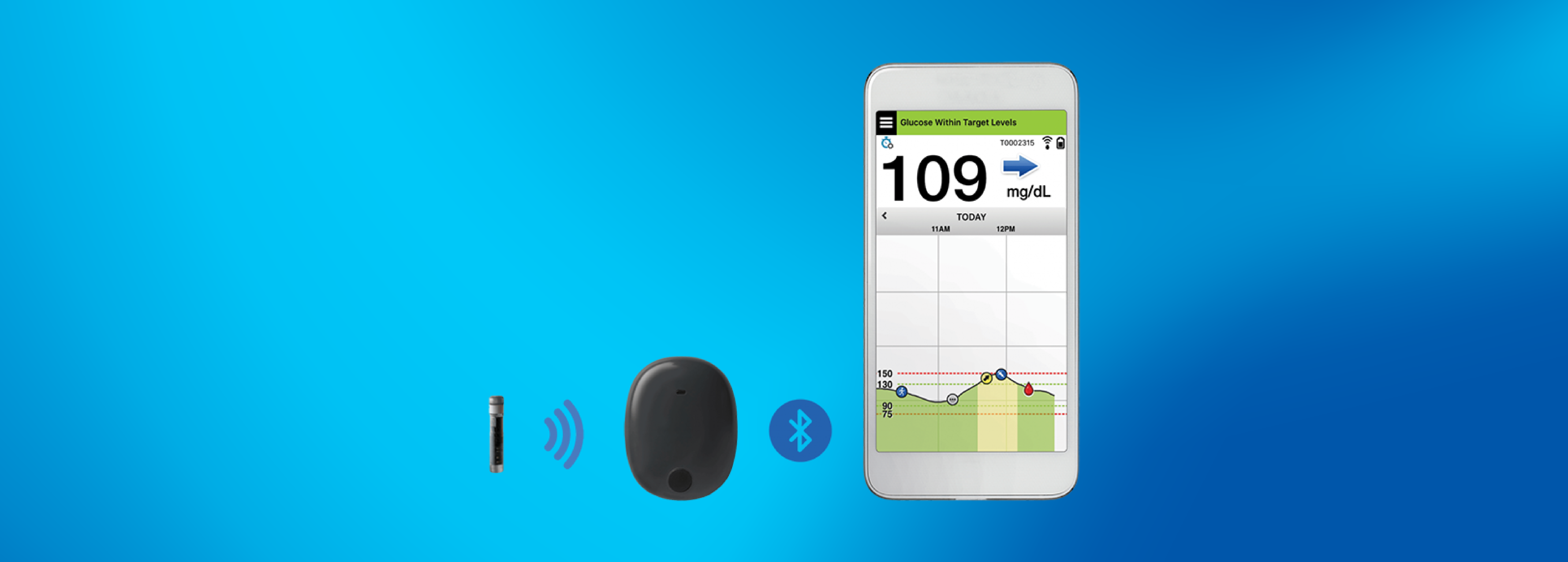
Beyond Type 1 - NOW APPROVED BY THE FDA - a new CGM is on the scene! The Eversense from Senseonics is the first implantable continuous glucose monitor. It lasts 90 days

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News