For People With Type 1 Diabetes, An 'Artificial Pancreas' Is Almost Here : Shots - Health News : NPR

OneTouch Vibe Plus Insulin Pump Earns FDA Approval And Health Canada License And Is First Pump Integrated With The Dexcom G5 Mobile Continuous Glucose Monitor

FDA approves Eversense E3 6-month continuous glucose monitor that requires fewer fingerstick blood glucose measurements - NotebookCheck.net News
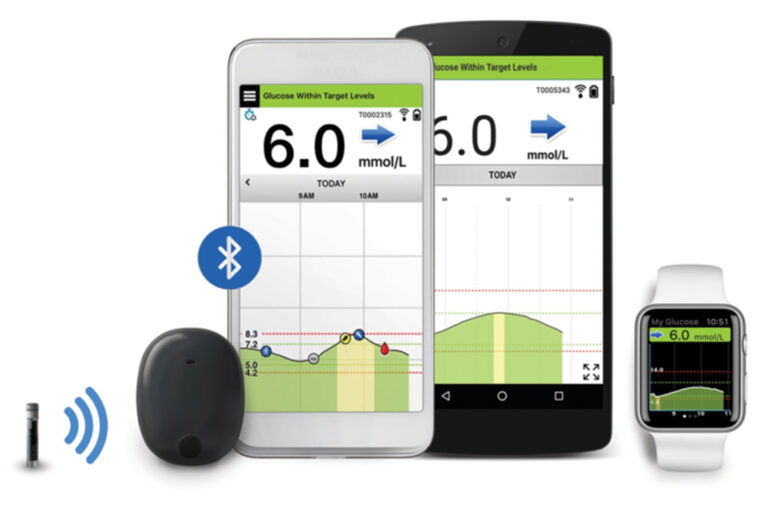
Eversense E3 CGM Approved for Two Sensors per Year: Your “Happily Ever(sense) After” - Taking Control Of Your Diabetes®
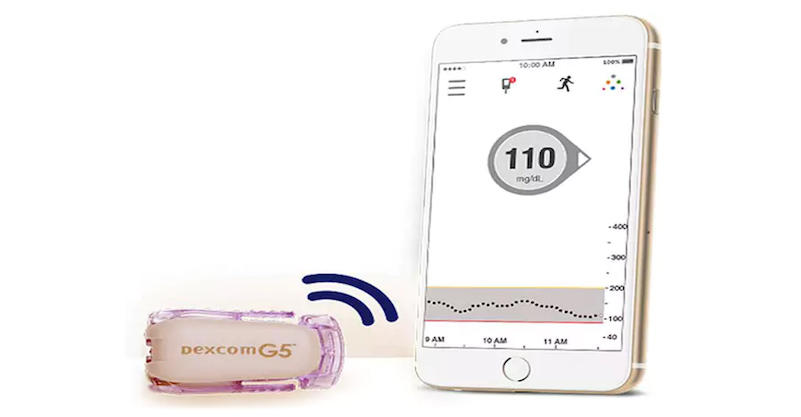
FDA approves diabetes device, Dexcom G5 Mobile CGM System, which aims to simplify blood sugar testing, dosing - CBS News




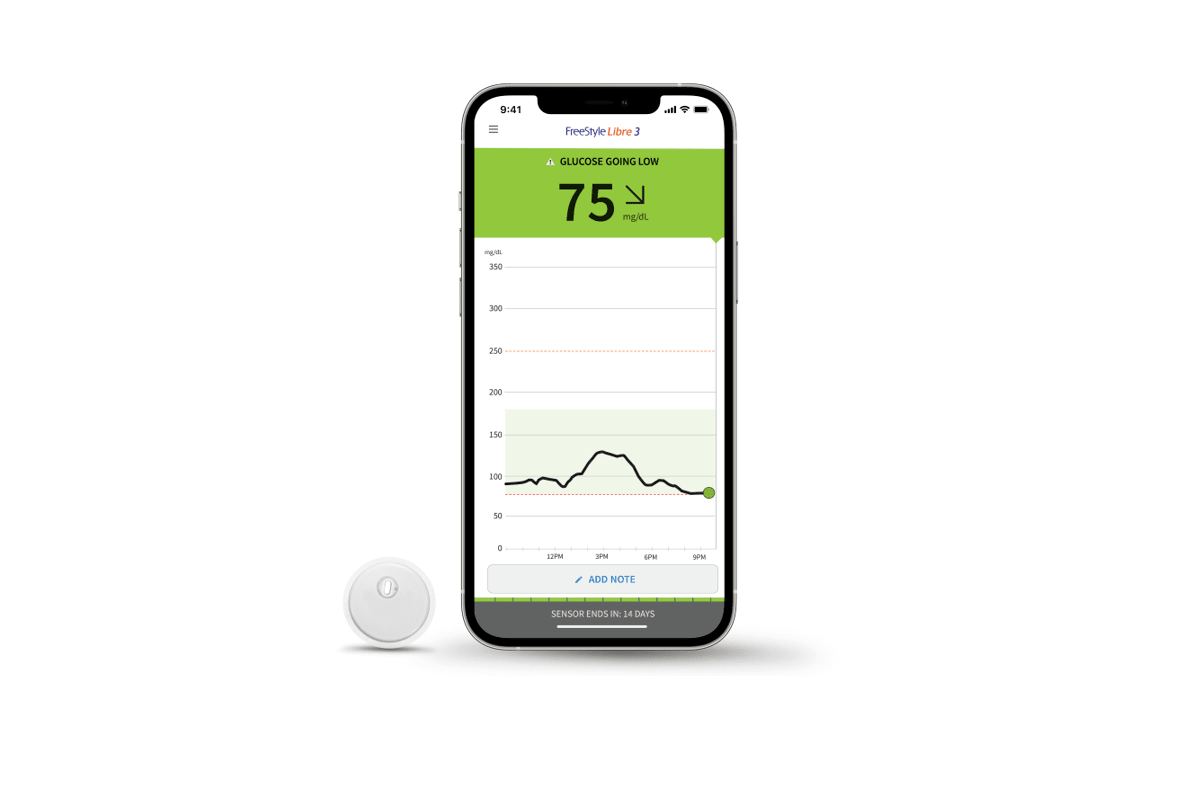
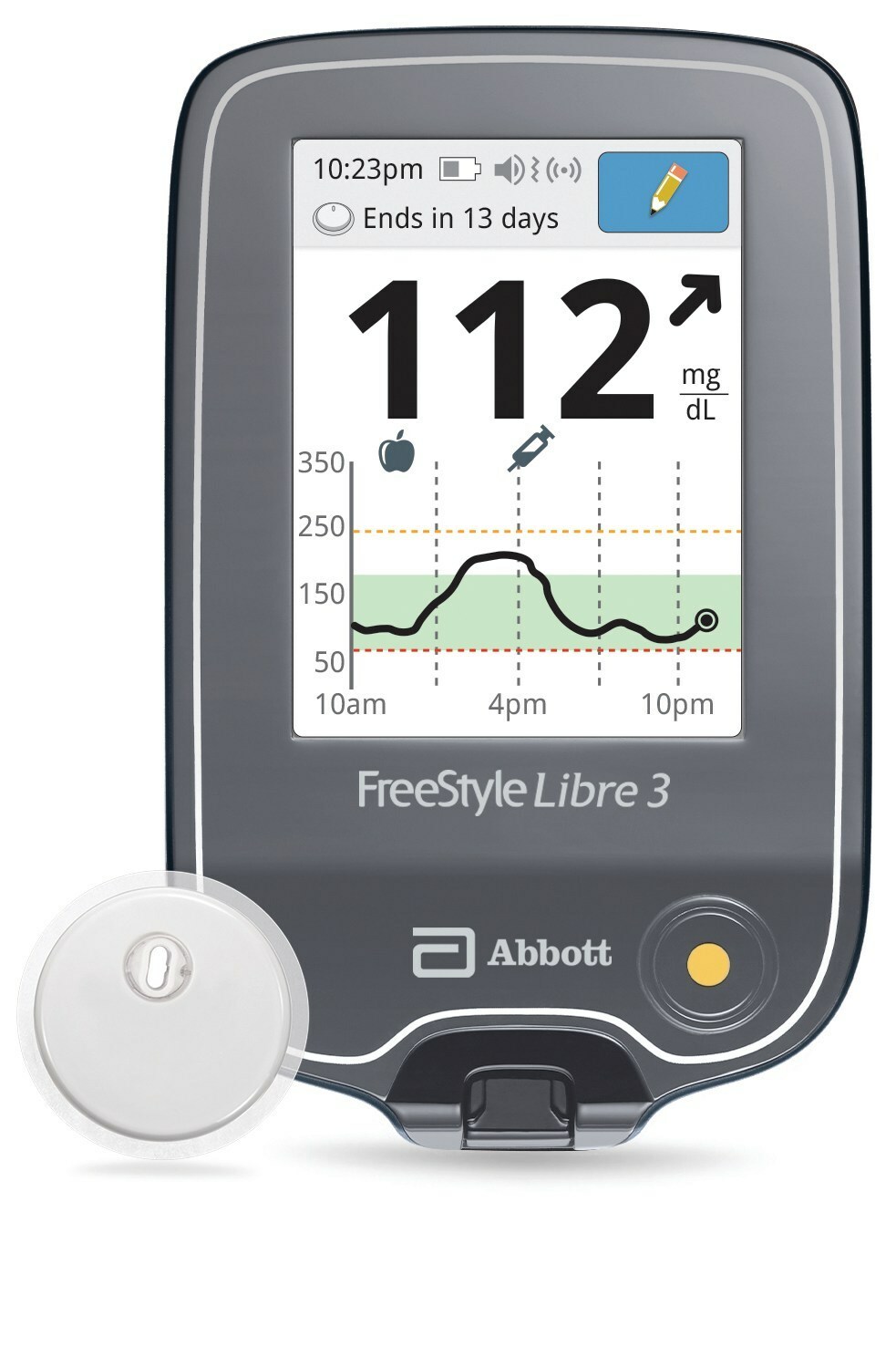
![Freestyle Libre 3 - [Pain-Free Blood] Glucose Monitoring - 2023 Freestyle Libre 3 - [Pain-Free Blood] Glucose Monitoring - 2023](https://cgmmonitors.com/wp-content/uploads/2023/01/All-You-Need-to-Know-About-Freestyle-Libre-3.jpg)